Different dietary patterns are associated with different microbiota composition profiles. Microbiota composition analyses of Western diets tend to report narrower bacterial diversity along with altered microbial profiles when compared to Mediterranean diets1. It is worth remembering that Western diets aren’t just “high in fat” or “carbohydrate-rich”, as they are often described.
In this fully referenced blog series on the human microbiome, our expert Miguel Toribio-Mateas discusses why the stability of our gut flora is as important to its diversity and shares his evidence-based clinical approach to gut health. You can read part 1 of this blog series here.
They feature a variety of what the NOVA food classification2 defines as “ultra-processed foods”, i.e. hyperpalatable, “energy-dense, high in unhealthy types of fat, refined starches, free sugars and salt, and poor sources of protein, dietary fibre and micronutrients”3. Typical ultra-processed food items are fizzy drinks, margarines and spreads, cookies, biscuits, breakfast cereals, energy bars, energy drinks, prepared pies, pizzas, meat nuggets and pre-packaged or “ready meals”, containing large amounts of saturated and trans-fats along with sucrose, emulsifiers, sweeteners and high glycaemic carbohydrates, e.g. maltodextrin, whilst offering little micronutrient value.
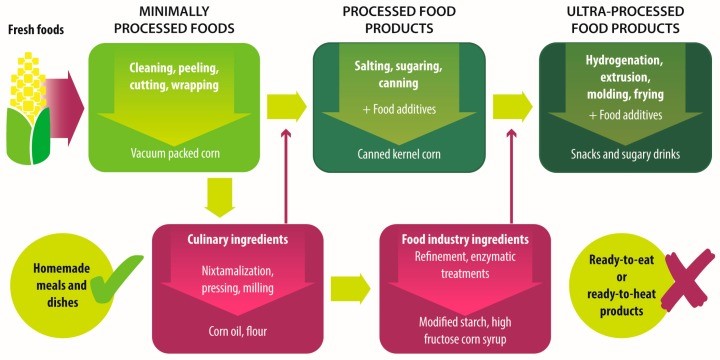
Demonising individual food items is not particularly helpful clinically. However, as the number of studies describing the effects of ultra-processed foods on the human gut microbiota continues to grow, practitioners should be able gain a better understanding of their patients’ health issues by becoming acquainted with their food shopping habits. For example, it is now known that dietary emulsifiers have been able to alter the mucus layer thickness, thereby affecting the interactions between gut microbes and host cells4 and mediating increased levels of pro-inflammatory lipopolysaccharide5. Sweeteners have been shown to induce metabolic aberrations mediated by alterations in gut microbiota in animal models6 and are known to reduce microbial diversity in humans7, leading to altered glucose homeostasis, decreased satiety, increased caloric consumption and weight gain8. Sweeteners can also affect cognitive processes such as memory, reward learning and taste perception9, all of which can affect an individual’s relationship with food as well as their food choices10. Interestingly, a Western dietary pattern has been seen to affect autonomic regulation and vagal-cardiac activity11, and reduced heart rate variability (HRV) – a sign of autonomic dysregulation – has been seen in individuals suffering from strong attachment to hyperpalatable foods, which some authors refer to as “food addiction”12,13.
The Mediterranean diet pattern as a source of richness and diversity
A growing number of studies of good methodological quality continue to provide substantiation for the ability of the Mediterranean Diet (MD henceforth) to effect positive changes in gut microbiota composition and diversity. On that basis, it is proposed that an MD pattern provides a reliable answer to the question of what “a healthy diet for the microbiome” might be.
As an example, gut microbiota and metabolome analysis of 51 vegetarians, 51 vegans and 51 omnivores distributed across four geographically distant cities in Italy by De Filippis et al.14. Researchers found that the adherence to the Mediterranean dietary pattern represented a vital factor contributing to the wider diversity of their gut microbiota. Adherence to the MD was assessed using the 11-unit dietary score by Agnoli et al.15 and the diversity and abundance of “healthy foods” such brightly coloured vegetables, fruit, nuts and minimally processed cereals consumed by participants was characterised using the Healthy Food Diversity (HFD) index by Drescher et al16. The clinical pearl provided by this study is that participants with the highest adherence level to the MD along with the highest HFD scores presented with highest levels of faecal short-chain fatty acids, irrespective of whether their diets included specific food items such as meat, fish of dairy14 . In practical terms, this makes the Mediterranean dietary pattern an exceptional versatile “template” that practitioners can individualise in order to meet patients’ satisfy their patients’ preferences. In the authors’ own clinical experience, using a practical tool such as the “50 food challenge” data collection chart17 can help boost patients’ creativity around food choices, thereby promoting engagement with the concept of dietary diversity and optimising compliance.
As another taster of the ample evidence substantiating the MD’s beneficial effect on the gut microbiota – in a transversal study of 31 adults without a previous diagnosis of cancer, autoimmune or digestive diseases – researchers at the Department of Functional Biology of the University of Oviedo in Asturias, Spain, found that participants with the closest adherence to a Mediterranean-style dietary pattern experienced statistically significant changes in a number of bacterial communities including overall higher abundance of Bacteroidetes, Prevotellacea and Prevotella, and a lower concentration of Firmicutes and Lachnospiraceae, assessed by 16S rRNA gene sequencing18. Adherence to the MD was defined as scores ≥ 4 in the validated Mediterranean Diet Score (MDS) by Trichopoulou et al.19. These alterations in gut microbiota richness and spread triggered by the MD are consistent with previously reported clinical data on the effects of increased dietary fibre from vegetables, legumes and whole – as in minimally processed – grains, as well as phenolic compounds and carotenoids typically featured in MD foods such as seasonal and citrus fruits, leafy, pod and root vegetables, in addition to bulbs, e.g. onions, garlic, leeks, etc. and not forgetting red wine and coffee20,21. Subjects with a MDS ≥ 4 also had higher concentrations of faecal the short chain fatty acids butyrate and propionate [18], measured by high performance liquid chromatography (HPLC).
The same research team reported a link between dietary bioactive compounds in the MD and the faecal metabolic phenolic profile of 74 healthy volunteers. According to Gutierrez-Diaz et al.22 participants with higher adherence to a MD dietary pattern (median MDS[1] ≥ 4) displayed higher levels of commensal Clostridia belonging to the cluster XVIa, particularly Faecalibacterium prausnitzii. This bundle of microbes from the Clostridia class falls under the Firmicutes phylum and is known for their ability to colonise the mucin layer of human colon, thereby aiding in the in the maintenance of gut homeostasis23. It includes species such as Eubacterium rectale, Papillibacter cinnamivorans, Eubacterium ventriosum, Butyrivibrio crossotus, Clostridium orbiscidens, Coprococcus eutactus, Roseburia intestinalis and Faecalibacterium prausnitzii (F. prausnitzii for short) known to plays a major role in mediating the production of butyrate from fermentable dietary carbohydrates.24,25. F. prausnitzii is considered to have strong anti-inflammatory properties26. This is largely mediated by its ability to produce butyrate, thereby protecting the gut mucosa27, but also by butyrate-independent pathways, which seem to include its ability to block NF-kappaB activation and IL-8 production28. Low levels of F. prausnitzii, Eubacterium rectale, and Eubacterium hallii have been associated with a peripheral inflammatory state in patients with cognitive impairment and brain amyloidosis29. Low levels of genus Roseburia microbes have been seen in patients with primary sclerosing cholangitis and ulcerative colitis (UC)30 as well as in those affected by constipation-predominant irritable bowel syndrome (C-IBD)31. In the first metagenome-wide study of gut microbiota in type 2 diabetes mellitus (T2D), researchers found that Roseburia intestinalis and F. prausnitzii concentrations were lower in T2D compared to healthy individuals32.
How to turn your gut into a Mediterranean gut
Regardless of the country you live in, gut bugs seen in all of these Mediterranean diet studies thrive when you eat a varied, colourful diet that contains as many colours from fresh foods as possible, on a daily basis. You’ll have heard me talk about “eating a rainbow” or “eating the rainbow” for years, and for as cute as this phrase is, it is also referring to the hard science of how dietary diversity feeds microbial diversity.
If you are a practitioner who uses stool tests, you will know that increasing the diversity of the foods you eat on a daily basis, i.e. minimising the repetition of foods, not only ensures you get a wider spread of nutrients but it also feeds a wider spread of your gut bugs, cultivating that all-important microbial diversity. If you’re unsure of how varied your diet really is, you should take the “50 food challenge”, which I’ve discussed previously with regards to whole-body health. Here’s a simple summary.
This is very simple:
- Download the “50 food challenge chart” by clicking on the links below.
- Keep track of every different food you eat for a week and aim for at least 50 foods, mostly plant-based and of all colours of the rainbow, the brighter the better.
- Do not exclude any groups of foods unless you actually need to for specific reasons that make you feel unwell.
- Note down every food only once only within the next 7 days to assess your diet’s diversity. For example, red and white onions count as 2 different foods, bread and pasta count as just one as they’re mostly made from the same ingredient, i.e. wheat (or whatever other ingredient, e.g. buckwheat). If you start filling out the chart on a Monday and you note down red onions as 1 ingredient, you may add white onions again on a Tuesday, but if you have red onions again anytime in the following 6 days, you can’t note them down again.
- As a rule of thumb, as fruit / veg / protein portion is the size of the palm of your hand. But even a tiny amount counts, which applies to herbs and spices as well as oils.
Some notes on using the 50 foods challenge chart
Remember that the idea is to increase the amount of foods you include in your diet, not to increase the amount of foods you exclude from your diet. Think about why you exclude foods and whether the rationale is valid or is just based on a belief rather than a fact. Working with a practitioner who understands your goals will help you get there more easily by drawing from their support.
If you’re just using the chart for yourself, please download in PDF format. If you’re a practitioner, here’s a PowerPoint file that I’ve used myself in clinical practice for years and that I am giving you copyright free to download and to customise with your own logo and credentials. This was published in the peer-reviewed journal Microorganisms, so ideally you should keep the reference:
Toribio-Mateas (2018) in Microorganisms 2018, 6(2), 35; https://doi.org/10.3390/microorganisms6020035.
I hope you and your clients find it useful!
Doing food challenges 2020 style
Biotech company Atlas Biomed specialising in microbiome testing Atlas Biomed has a free app that allows you to take a photo of your food plate and to identify the ingredients via artificial intelligence so that they go on your food diary. The app can be downloaded free of charge, and if you take an Atlas test, you’ll see your results, as well as your food diary all in the same place. A very cool feature for practitioners is having access to your client’s food diaries from your practitioner account. This means no more note taking or filling out of long diet diary questionnaires. Simply ask your clients to take a pic of their meals, and you’ll see them immediately. Not only that, the colours and ingredients identified by the app help your food recommendations be more precise. Being a natural born geek, I find this absolutely fascinating, and believe that this is where the future of personalised health is. Links for the app are here: Apple and Android .
About Miguel
Miguel Toribio-Mateas is a clinical neuroscientist and nutrition practitioner, currently working towards a professional doctorate in health neuroscience, using health-related quality of life outcome measures alongside biomarkers from microbiome analysis to assess the complex effects of food on cognitive function and mental health via the gut brain axis.
With many thanks to Miguel for this blog. If you have any questions regarding the health topics that have been raised, please don’t hesitate to get in touch with Amanda via e-mail or phone:
amanda@cytoplan.co.uk
01684 310099
Amanda Williams and the Cytoplan Editorial Team
References
- Garcia-Mantrana, I., et al., Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front Microbiol, 2018. 9: p. 890.
- Monteiro, C.A., et al., Ultra-processing. An odd ‘appraisal’. Public Health Nutr, 2018. 21(3): p. 497-501.
- Monteiro, C.A., et al., The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr, 2018. 21(1): p. 5-17.
- Cani, P.D. and A. Everard, Keeping gut lining at bay: impact of emulsifiers. Trends Endocrinol Metab, 2015. 26(6): p. 273-4.
- Chassaing, B., et al., Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut, 2017. 66(8): p. 1414-1427.
- Suez, J., et al., Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature, 2014. 514(7521): p. 181-6.
- Frankenfeld, C.L., et al., High-intensity sweetener consumption and gut microbiome content and predicted gene function in a cross-sectional study of adults in the United States. Ann Epidemiol, 2015. 25(10): p. 736-42.e4.
- Pearlman, M., J. Obert, and L. Casey, The Association Between Artificial Sweeteners and Obesity. Curr Gastroenterol Rep, 2017. 19(12): p. 64.
- Burke, M.V. and D.M. Small, Physiological mechanisms by which non-nutritive sweeteners may impact body weight and metabolism. Physiol Behav, 2015. 152(Pt B): p. 381-8.
- Creze, C., et al., The Impact of Caloric and Non-Caloric Sweeteners on Food Intake and Brain Responses to Food: A Randomized Crossover Controlled Trial in Healthy Humans. Nutrients, 2018. 10(5).
- Kim, J.Y., et al., Eating Habits and Food Additive Intakes Are Associated with Emotional States Based on EEG and HRV in Healthy Korean Children and Adolescents. J Am Coll Nutr, 2017. 36(5): p. 335-341.
- Meule, A. and A. Kubler, Food cravings in food addiction: the distinct role of positive reinforcement. Eat Behav, 2012. 13(3): p. 252-5.
- Young, H.A. and D. Benton, Heart-rate variability: a biomarker to study the influence of nutrition on physiological and psychological health? Behav Pharmacol, 2018. 29(2 and 3 – Special Issue): p. 140-151.
- De Filippis, F., et al., High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut, 2016. 65(11): p. 1812-1821.
- Agnoli, C., et al., A Priori–Defined Dietary Patterns Are Associated with Reduced Risk of Stroke in a Large Italian Cohort. The Journal of Nutrition, 2011. 141(8): p. 1552-1558.
- Drescher, L.S., S. Thiele, and G.B. Mensink, A new index to measure healthy food diversity better reflects a healthy diet than traditional measures. J Nutr, 2007. 137(3): p. 647-51.
- Toribio-Mateas, M., Harnessing the Power of Microbiome Assessment Tools as Part of Neuroprotective Nutrition and Lifestyle Medicine Interventions. Microorganisms, 2018. 6(2): p. 35.
- Gutierrez-Diaz, I., et al., Mediterranean diet and faecal microbiota: a transversal study. Food Funct, 2016. 7(5): p. 2347-56.
- Trichopoulou, A., et al., Diet and overall survival in elderly people. Bmj, 1995. 311(7018): p. 1457-60.
- Tap, J., et al., Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ Microbiol, 2015. 17(12): p. 4954-64.
- Gonzalez, S., et al., Dietary intake of polyphenols and major food sources in an institutionalised elderly population. J Hum Nutr Diet, 2014. 27(2): p. 176-83.
- Gutierrez-Diaz, I., et al., Adherence to a Mediterranean Diet Influences the Fecal Metabolic Profile of Microbial-Derived Phenolics in a Spanish Cohort of Middle-Age and Older People. J Agric Food Chem, 2017. 65(3): p. 586-595.
- Lopetuso, L.R., et al., Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathogens, 2013. 5: p. 23-23.
- Van den Abbeele, P., et al., Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. Isme j, 2013. 7(5): p. 949-61.
- El Aidy, S., et al., Intestinal colonization: how key microbial players become established in this dynamic process: microbial metabolic activities and the interplay between the host and microbes. Bioessays, 2013. 35(10): p. 913-23.
- Lopez-Siles, M., et al., Faecalibacterium prausnitzii: from microbiology to diagnostics and prognostics. Isme j, 2017. 11(4): p. 841-852.
- Sokol, H., et al., Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm Bowel Dis, 2009. 15(8): p. 1183-9.
- Sokol, H., et al., Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A, 2008. 105.
- Cattaneo, A., et al., Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging, 2017. 49: p. 60-68.
- Bajer, L., et al., Distinct gut microbiota profiles in patients with primary sclerosing cholangitis and ulcerative colitis. World J Gastroenterol, 2017. 23(25): p. 4548-4558.
- Gobert, A.P., et al., The human intestinal microbiota of constipated-predominant irritable bowel syndrome patients exhibits anti-inflammatory properties. Sci Rep, 2016. 6: p. 39399.
- Qin, J., et al., A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature, 2012. 490.
- Estruch, R., et al., Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med, 2013. 368(14): p. 1279-90.
- Guasch-Ferre, M., et al., The PREDIMED trial, Mediterranean diet and health outcomes: How strong is the evidence? Nutr Metab Cardiovasc Dis, 2017. 27(7): p. 624-632.
- Martínez-González, M.A., et al., Benefits of the Mediterranean Diet: Insights From the PREDIMED Study. Progress in Cardiovascular Diseases, 2015. 58(1): p. 50-60.
- Estruch, R., et al., Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. The Lancet Diabetes & Endocrinology, 2016. 4(8): p. 666-676.
- Martinez-Lapiscina, E.H., et al., Virgin olive oil supplementation and long-term cognition: the PREDIMED-NAVARRA randomized, trial. J Nutr Health Aging, 2013. 17(6): p. 544-52.
- Martínez-Lapiscina, E.H., et al., Mediterranean diet improves cognition: the PREDIMED-NAVARRA randomised trial. 2013. 84(12): p. 1318-1325.
- Valls-Pedret, C., et al., Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern Med, 2015. 175(7): p. 1094-103.
- Toledo, E., et al., Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern Med, 2015. 175(11): p. 1752-60.
- Babio, N., et al., Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. 2014. 186(17): p. E649-E657.
- Holscher, H.D., et al., Walnut Consumption Alters the Gastrointestinal Microbiota, Microbially Derived Secondary Bile Acids, and Health Markers in Healthy Adults: A Randomized Controlled Trial. J Nutr, 2018. 148(6): p. 861-867.
- Bamberger, C., et al., A Walnut-Enriched Diet Affects Gut Microbiome in Healthy Caucasian Subjects: A Randomized, Controlled Trial. Nutrients, 2018. 10(2).
- de Souza, R.G.M., et al., Nuts and Human Health Outcomes: A Systematic Review. Nutrients, 2017. 9(12).
- Lamuel-Raventos, R.M. and M.-P.S. Onge, Prebiotic nut compounds and human microbiota. Critical Reviews in Food Science and Nutrition, 2017. 57(14): p. 3154-3163.
- Holscher, H.D., et al., Almond Consumption and Processing Affects the Composition of the Gastrointestinal Microbiota of Healthy Adult Men and Women: A Randomized Controlled Trial. Nutrients, 2018. 10(2).
Last updated on 8th March 2021 by cytoffice



During this COVID epidemic how much Vit C should I take? At the moment I’m having 3 gms of cytoplan .
Hi Liz – I tend to prefer the food based Vitamin C products which are more gentle and are effective at lower doses as they are retained by the body. If you are going for a food based Vit C products such as cherry C then 4 capsules a day (i.e. 800mg) is a very good dose in conjunction with a healthful diet, for optimal health and protection. It is best to spread the dose out throughout the day. If you choose to take ascorbic acid then it is needed at higher doses, around 1-2grams /day or to bowel tolerance if less. Thanks, Amanda