“The science of gene-diet interaction is one of the most promising strategies we have in order to improve general health”, a quotation from Professor Giovanni Scapagnini taken from this week’s blog, in which clinical neuroscientist and functional nutrition practitioner Miguel Toribio-Mateas interviews Professor Scapagnini on the complex subject of nutrigenetics and its role in disease prevention and treatment.
Professor Scapagnini, an Italian medical doctor and author of 250 indexed papers, will be speaking at our Cytoplan Practitioner Event on Saturday 25th February. To find out more about the event and to book your place, please follow this link.
An introduction from Miguel
Today I have the pleasure to interview Professor Giovanni Scapagnini, an Italian medical doctor and Ph.D. in Neurobiology with international expertise in surgical research, having worked at the Northwick Park Institute for Medical Research in the UK in the late 90s, and at the Laboratory of Adaptive Systems, National Institute of Neurological Disorders and Stroke, National Institute of Health in Bethesda, MD, USA in the 2000s.
Prof. Scapagnini currently holds two academic positions as Assistant Professor with the Institute of Neurological Sciences, Italian National Research Council and with Blanchette Rockefeller Neurosciences Institute, West Virginia University. He has recently obtained a visiting professorship with the Institute of Human Virology, University of Maryland, where he is in charge of a research project on HIV dementia. He is also the scientific director of the “Research & Progress” foundation, founded by Dr Robert C. Gallo.
He is author of nearly 250 indexed scientific papers and several book chapters. His fields of research regard gene expression profiles of cellular stress response and biology and molecular mechanisms of brain aging and nerurodegenerative disorders. In particular he has studied the anti-aging activities of several nutraceuticals present in the Mediterranean diet.
I have been a follower of Prof. Scapagnini’s work for quite some time, so I am delighted to have the chance to interview him about the all-important subject of Nutrigenetics.
Prof. Scapagnini, how would you define nutrigenetics “in plain English” to a practitioner audience interested in the area?
Two terms invariably come up when discussing this subject, namely nutrigenetics and nutrigenomics. Modern nutrition has revealed much about the ways in which individual genetic traits or genotypes modulate the responses to dietary factors.
This is the branch of scientific study known as Nutrigenetics. Complementarily, the discipline that provides rich mechanistic insights into how nutrients and other food components regulate gene expression as well as cell and tissue functions is considered as Nutrigenomics. Thus, Nutrigenetics and Nutrigenomics are defined as the science of the effects of genetic variation on dietary responses and the role of nutrients and bioactive food compounds on gene expression, respectively.
It is important to note that both terms are closely related but not interchangeable. Nutrigenetics research involves genetic inheritance and its variations in the response to nutrients and dietary patterns, whereas nutrigenomics investigations focus on dietary effects on genome stability, epigenome alterations, RNA and miRNA alterations, protein expression and metabolite changes.
Thank you. Nutrigenetics / nutrigenomics is a booming research area with complex ramifications into health and disease, and the cornerstone of individualised nutrition. What is your view regarding the importance of nutrigenetic assessment (aka testing) in age-related conditions?
As you mention, a “healthy balanced diet” is relied upon as the cornerstone of public health policies for reducing the risk of non-communicable conditions such as obesity, cardiovascular disease, and related morbidities.
However, several gene–diet interactions can significantly affect the quality of ageing, and depending on these you can age more or less successfully, developing more or fewer age-related chronic diseases. Technological advances have now driven down the costs and improved the reliability and availability of personal genome testing, allowing not just personalisation of nutritional recommendations, but its individualisation.
Armed with genomic information along with functional test results, a functional medicine / nutrition / dietetics practitioner is now able to truly individualise his/her nutritional recommendations, providing a programme that may be different to the accepted public health guidelines which are modelled to be good for population averages, but haven’t been designed to be used prescriptively by individuals.
By basing their recommendations on genetic and functional data, practitioners are increasing their clients’ chances of experiencing better health outcome and better, more successful ageing.
This is very interesting. Practitioners often ask me about genetic testing and my answer is always the same. Without functional data to marry up with genetic variation, a genetic test is just an academic exercise. But by combining genetics with functional data you could potentially have access to a comprehensive picture of an individual’s health. I say “potentially” because there are many genetic tests out there that analyse anything from a handful of genes to hundreds of them, and practitioners are often confused as to the suitability of certain tests to assess their clients’ health concerns. What is your expert opinion on how nutrigenetics can be used for disease prevention and treatment?
What you just said is key. The perfect example is the MTHFR (methylenetetrahydrofolate reductase) gene, which I know has gained a fair amount of interest amongst functional medicine practitioners. Knowing about variation from the norm, known as SNPs (single nucleotide polymorphisms) in this gene is interesting, but pretty meaningless unless you are also aware of that person’s serum homocysteine levels. And there are many other examples like this.
The wrong way to go about nutrigenetics is to “prescribe a genetic diet” or a nutritional programme based solely on someone’s genetic variation. Additionally, genes never change, whilst their expression and the metabolites associated with them do. You can’t improve what you can’t measure, so if the only measurement you have is genetic, you can’t improve your patient’s health at all.
On the other hand, if you have functional measurements, e.g. homocysteine levels, coupled perhaps with methyl donor B vitamins such as folate and B12, then you’ve got a much better chance to improve your patient’s health outcomes, and to obtain evidence that this is the case by measuring these at baseline and after a few weeks or months.
I couldn’t agree more, and this is precisely how I practise myself, measuring again 3 months after the first reading and assessing change, then discussing what implications that change has to my client’s programme.
As a medical doctor that makes total sense to me. Unfortunately tests carry a cost and they’re only available privately, but I believe within a few years technology will make them available at a fraction of their cost today so an increasing number of people can afford to follow the approach you suggest. I believe that is where the future of medicine lies.
There is convincing evidence that the risk of common diet-related diseases, such as cardiovascular disease (CVD), type 2 diabetes, osteoporosis, dementia, and some cancers, is influenced by genetic factors, and that carrying specific genetic variants (which some people incorrectly used to call “mutations”) can modulate individual biological responses to nutrients.
This is just one of the many important aspects to look at, in order to better define a tailored nutrition programme. Besides the Nutrigenetics aspects, diet is a complex variable that interacts with other environmental factors, like exposure to toxins. This takes place at several biochemical levels, throughout the individual’s lifespan. It is always fundamental to match genetic susceptibility with phenotype or presentation, and to fit lifestyle, behavioural and functional factors into the picture, in order to better understand what the best nutritional interventions are for that specific person.
Coming back to your MTHFR example, one of the most widely studied associations between gene polymorphisms, nutrients and a risk biomarkers, is that between the MTHFR gene C677T polymorphism, folic acid (or folate), and homocysteine. The 677T version of the enzyme has only about 35 % of the activity of the 677C version. A low folate status leads to high homocysteine levels in TT individuals.
As an example, this means that 200μg of folic acid has been shown in many trials to be insufficient to maintain homocysteine levels below the risk level in this genetic group. Homocysteine is accepted as an independent risk factor for cardiovascular and other diseases. It is accepted that increasing folate intake to 400–600μg per day will keep homocysteine levels below the risk level in most TT individuals. Thus, in individuals carrying two copies of the “slow” enzyme, there is a clear evidence for functional medicine / nutrition / dietetic practitioners that they need to look at serum homocysteine levels as well as variants in the MTHFR gene, and to support their patients accordingly, with higher amounts of folate if needed.
Some reputable genetic tests – and I base my opinion on reputability on how well studied the reported genetic variants are – include polymorphisms related to the metabolism of vitamins, e.g. vitamin A, E, C, etc. These relate to difficulty in the absorption and transport of such essential micronutrients. In these cases, it is important to verify the reduction of vitamin levels in the blood, support the person with a individualised levels of nutrient supplementation.
There’s a lot of talk about the metabolic effects of fructose. Is there any way to assess a genetic susceptibility for an individual’s sensitivity to fructose?
The test is associated to the identification of person carriers of polymorphisms of the Aldolase-B gene, that when present in homozygotes form, are responsible for the Hereditary Fructose Intolerance, a very serious genetic disease, with complex metabolic defects. It is not to be confused with fructose malabsorption, a form of fructose “intolerance”, a digestive disorder.
Thank you. As we’re just starting the year, many go onto “detox diets” trying to “purge” the excess of the Christmas period. Because of the misconception of what detoxification means, some have trivialised this all-important process and often doubt the professional reputability of those who acknowledge it. As a geneticist, molecular biologist, and medical doctor, what are your views on the management of a person’s detoxification capacity by means of nutritional interventions?
I know that many scientists dismiss the detoxification process as they associate it with faddish “detox diets”, pills or teas. Obviously these are just gimmicks, so you can understand the origin of the misconception, despite the huge evidence base documenting cellular detoxification processes.
From a Biochemical point of view, detoxification is a fundamental enzymatic process that enables the elimination of potentially toxic xenobiotics from an organism. Detoxification – call it “detox” if you’d like – is based on two major systems, type I (.i.e. 1), at hepatic level, and played by cytochromes, and type II (i.e. 2), at hepatic and also at several cellular level, owing its name to detoxifying enzymes of type 2.
Several gene polymorphisms are present for most of these detoxifying enzymes in general populations and this genetic variability means that the detoxifying capacity of every individual is different. Some of these systems, mostly type II detoxification, can be properly upregulated and stimulated by several nutraceuticals. This is the case of cruciferous vegetables, which have been shown to be able to reduce cancer risk in carriers of null GSTT1 and GSTM1 genes, two variants of the glutathione S transferase, a key detoxifying enzyme. A number of genes are involved in effective cellular detoxification, the process that takes place 24 hours a day, every day of your life. These include enzyme encoding genes such as SOD (superoxide dismutase) and NAT (N-acetyltransferase), to name but a couple.
There is plenty of scientific evidence of how numerous nutrients have the power to either induce or inhibit detoxification enzymes (sometimes both!). Undoubtedly practitioners who are have the knowledge to introduce these nutrients in their patients’ regimes have an edge on those who don’t, as effective cellular detoxification is key to avoid genomic instability, one of the major driving forces towards cellular senescence and dysfunction.
Thank you so much Prof. Scapagnini for dispelling myths. As a matter of fact, BANT (the British Association for Applied Nutrition and Nutritional Therapy) has just released a “Detox4Life” educational poster for its members that encapsulates many of the points you covered in your explanation about detoxification.
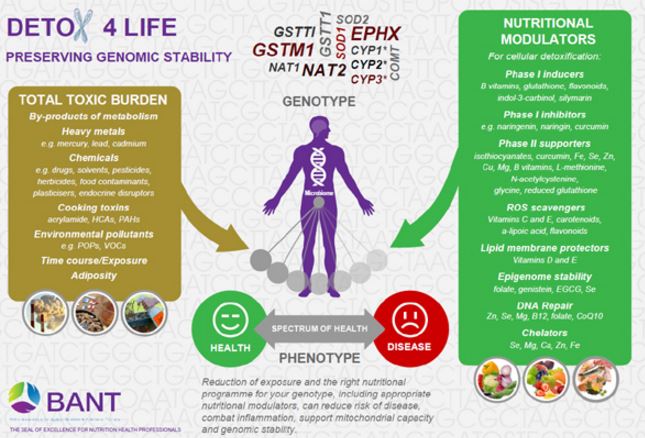
To download this poster, please follow this link to the BANT website.
I’m sure readers would love to deepen their knowledge of the points we’ve discussed today. Are there any key papers you could refer us to?
Thank you Miguel for your questions, it’s been a pleasure answering them! I’d like to emphasise how diet and bioactive components present in food and nutraceuticals are extremely powerful daily weapons to modulate cellular gene expression, activate adaptation systems, and favour healthy longevity. The science of gene-diet interaction is one of the most promising strategies we have in order to improve general health. I look forward to seeing you and many of your colleagues at the event on 25th February. Here are my top 3 reads on the subjects. I’ll be sharing many more with you on the day as part of the study pack.
Miguel Toribio-Mateas
 Miguel is a nutrition practitioner, author and researcher with extensive knowledge and expertise in functional medicine and ageing science. Miguel completed a MSc in Clinical Neuroscience at Roehampton University in 2016 and was awarded a scholarship for multidisciplinary doctoral research in brain ageing and cognitive decline by Santander Bank, assessing the clinical application of the Bredesen Protocol under the auspices of the Institute for Work Based Learning at Middlesex University.
Miguel is a nutrition practitioner, author and researcher with extensive knowledge and expertise in functional medicine and ageing science. Miguel completed a MSc in Clinical Neuroscience at Roehampton University in 2016 and was awarded a scholarship for multidisciplinary doctoral research in brain ageing and cognitive decline by Santander Bank, assessing the clinical application of the Bredesen Protocol under the auspices of the Institute for Work Based Learning at Middlesex University.
Professor Giovanni Scapagnini
 Currently an Associate Professor of Clinical Biochemistry and Molecular Biology at Università of Molise, Italy. He was previously employed as an Assistant Professor at the Blanchette Rockefeller Neurosciences Institute, West Virginia University, Rockville, MD and at the Institute of Neurological Sciences, Italian National Research Council. He also worked as a Visiting Scientist at the NINDS, National Institute of Health, Bethesda, MD, and at the Northwick Park Institute for Medical Research, Harrow, UK. Since 2004, he is a Visiting Professor at the University of Maryland (IHV), Baltimore, MD. Dr. Scapagnini is a founder and member of the Board of Directors of the Italian Society of Nutraceuticals SINUT. His current work focuses on the functional roles of food phytochemicals as redox regulators of aging process.
Currently an Associate Professor of Clinical Biochemistry and Molecular Biology at Università of Molise, Italy. He was previously employed as an Assistant Professor at the Blanchette Rockefeller Neurosciences Institute, West Virginia University, Rockville, MD and at the Institute of Neurological Sciences, Italian National Research Council. He also worked as a Visiting Scientist at the NINDS, National Institute of Health, Bethesda, MD, and at the Northwick Park Institute for Medical Research, Harrow, UK. Since 2004, he is a Visiting Professor at the University of Maryland (IHV), Baltimore, MD. Dr. Scapagnini is a founder and member of the Board of Directors of the Italian Society of Nutraceuticals SINUT. His current work focuses on the functional roles of food phytochemicals as redox regulators of aging process.
A Cytoplan Practitioner Education Day
After each of the 2 keynote lectures, the session will turn into a workshop facilitated by Miguel. To find out more about this event and to book your place, please follow this link.
With many thanks to Miguel and Professor Scapagnini for this Q & A session, if you have any questions regarding the health topics that have been raised please don’t hesitate to get in touch with me (Amanda) via phone; 01684 310099 or e-mail (amanda@cytoplan.co.uk).
Amanda Williams and the Cytoplan Editorial Team: Miguel Toribio-Mateas and Joseph Forsyth.
References
Davinelli S, Willcox DC, Scapagnini G. (2012) Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immun Ageing. 2012 Apr 23;9:9. Available from: https://www.ncbi.nlm.nih.gov/pubmed/22524452
McCann SE, Roberts MR, Platek ME et al (2010) Nutrigenetics: the relevance of polymorphisms. In: Milner JA, Romagnolo DF
(eds) Bioactive compounds and cancer. Humana Press, Springer, New York, pp 71–99. Available from http://link.springer.com/chapter/10.1007%2F978-1-60761-627-6_4.
Aiello, A., Accardi, G., Candore, G., Carruba, G., Davinelli, S., Passarino, G., Scapagnini, G., Vasto, S. & Caruso, C. (2016). Nutrigerontology: a key for achieving successful ageing and longevity. Immun Ageing, 13, 17. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27213002
Last updated on 31st January 2025 by cytoffice




Thank you Cytoplan! This was a fascinating interview and a confirmation that the future need not be bleak for people like me with several polymorphisms. The science of Nutrigenetics gives one back some semblance of control over one’s health issues.
Deborah Tayler
Fascinating, exciting, awesome! Great interview!