Methylation is a key enzymatic process that occurs billions of times a second in every human cell and is important for the proper function of all body systems. In our latest blog, we deep dive into what it is and it’s importance for health, well-being and energy.
What is methylation? 1-5
Methylation is a key enzymatic process that occurs billions of times a second in every human cell and is important for the proper function of all body systems. Simply put, methylation is the process of transferring a methyl group, which is made up of 1 carbon atom and 3 hydrogen atoms, to and from various molecules in the body.
This process is crucial in regulating various biochemical functions, including DNA synthesis, gene expression, neurotransmitter production, detoxification, homocysteine regulation, and much more!
Methyl donors
Methylation requires methyl donors which are nutrients that play an important role in single-carbon metabolism as they can donate a methyl group to another compound, altering its activity.
Key methyl donors include:
- folate
- vitamins B2, B6, and B12
- zinc
- choline
- and betaine, also known as trimethylglycine (TMG).
Methionine is another important substrate and serves as a precursor to S-Adenosyl-L-methionine (SAMe), a vital universal methyl donor that donates methyl groups to methyltransferase enzymes that support a diverse range of methylation-dependent processes.
Methylation, homocysteine and glutathione
An important function of methylation, which interests many practitioners, is the capacity to metabolise the amino acid homocysteine. Homocysteine is produced during the conversion of methionine to SAMe in the methionine cycle and relies on vitamin-derived cofactors such as vitamins B6, B12, and folate. While we need some homocysteine to make proteins in the body, in elevated amounts it becomes an inflammatory substance that can promote blood clot formation and negatively affect endothelial and therefore, cardiovascular health. During methylation, homocysteine can either be remethylated back to methionine by the acceptance of a methyl group or be converted into cysteine via the transsulfuration pathway, which is important in the production of the antioxidant glutathione.
Methylation dysfunction
Methylation is a complex biochemical process that can easily be disrupted by our internal and external environment, including nutrient intake, stress, sleep, and gut health. Dysregulation has been linked to various health conditions such as Alzheimer’s disease5, depression6, anxiety7, osteoporosis8, and cardiovascular disease.1-5
This article offers an overview of methylation and how nutrition and lifestyle factors may play a role in its dysfunction. Methylation can seem complex in clinical practice, with an interplay of individual differences and single-nucleotide polymorphisms (SNPs) playing a huge role in a person’s methylation capacity. A holistic individualised approach, often including functional and genetic testing, may be needed to fully support individuals, rather than a one-size-fits-all protocol.
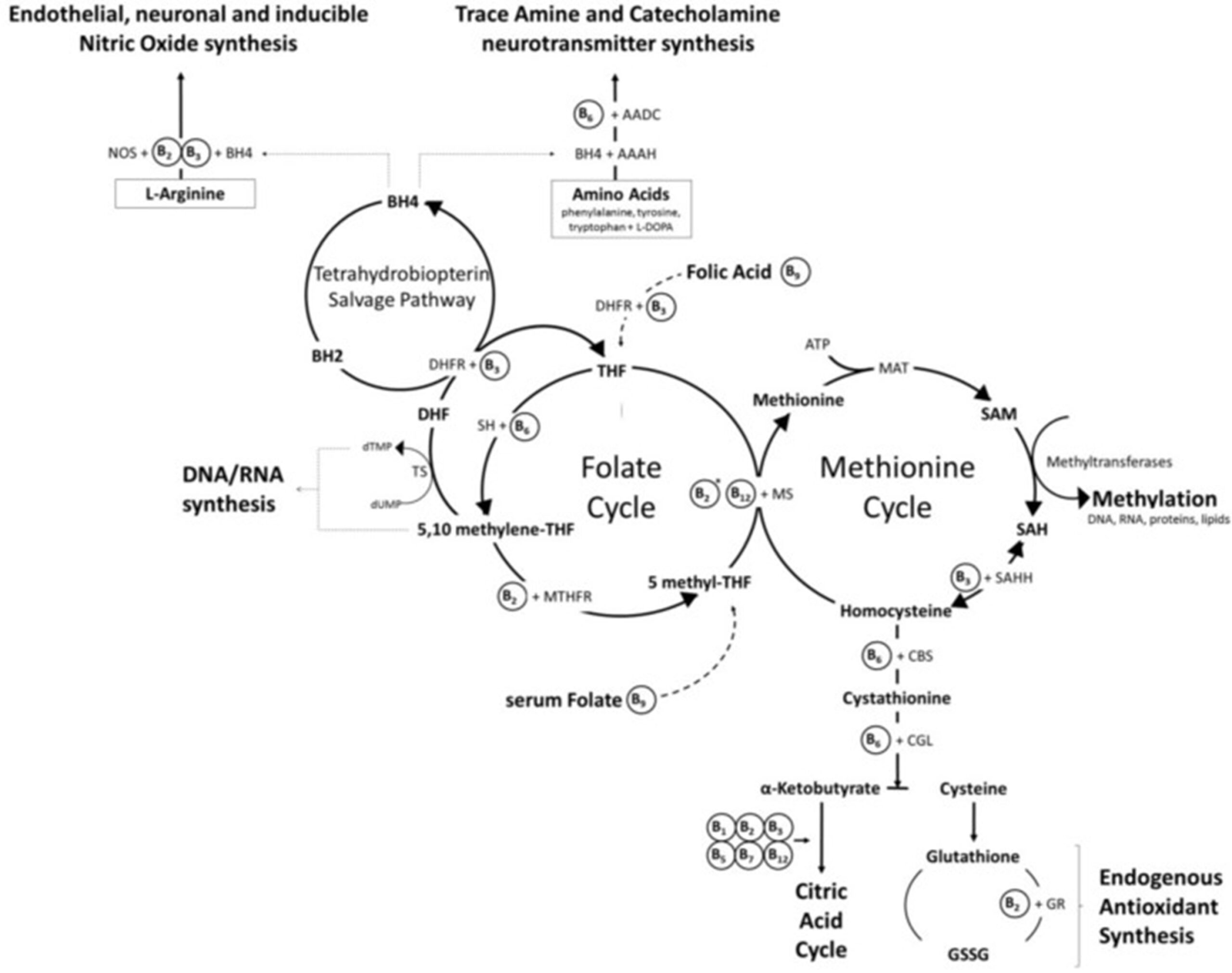
Diagram3
Why do we need methylation? 1-4
Methylation significantly influences every aspect of health. It plays a vital role in all biological systems and is part of:
- Hormone production and metabolism
- One of the phase 2 detoxification pathways
- Glutathione production
- DNA synthesis, epigenetic modulation, and gene expression
- The synthesis and breakdown of neurotransmitters
- Histamine breakdown
- ATP, carnitine, and COQ10 production
- The immune response process
- Cardiovascular health via its role in homocysteine metabolism, production of COQ110 and nitric oxide
- Structural integrity of DNA, phospholipids, myelin, and bone
- Bile formation
- Synthesis of phosphatidylcholine; a major component of myelin sheath surrounding neurons
- Circadian rhythm regulation
Methylation and energy production 9-12
There are many ways in which methylation can impact energy production and when impaired, can lead to tiredness and fatigue.
At a basic level, low energy can often be a symptom of poor nutrient intake. A lack of just one of the vital methyl donor nutrients, such as B12 or folate, can impact methylation and cause dysfunction, affecting optimal health and vitality.
B vitamins
B vitamins are involved in every aspect of generating energy; they act as essential co-enzymes in aerobic respiration in the mitochondria and play a direct role in the citric acid cycle, the electron transport chain, and the formation of adenosine triphosphate (ATP). The metabolically active form of B6, pyridoxal 5′-phosphate (P5P), acts as a cofactor for enzymes involved in amino acid metabolism, glycogenesis, and gluconeogenesis to support blood sugar balance, metabolic function, and energy homeostasis.
Folate
Folate as the active 5-methyltetrahydrofolate (5-MTHF) is needed for nitric oxide synthesis that directly supports vasodilation, blood pressure regulation, and mitochondrial biogenesis. Mitochondria are the powerhouse of our cells that are responsible for the production of ATP: the more mitochondria we have the better we are at producing ATP for energy.
CoQ10
Methylation itself produces various substances needed for optimal energy production. This includes CoQ10, an antioxidant that has a role in the electron transport chain and oxidative phosphorylation.11 It is involved in the synthesis of creatine, another important substrate used for ATP recycling, and the phosphocreatine system, making it vital for producing energy in the skeletal muscles during physical activity.
Carnitine
Carnitine, an amino acid also generated by methylation, supports the shuttling of fats across the cell membrane to be used as a fuel source.
Tyrosine
Another amino acid to consider concerning energy production is tyrosine. Tyrosine synthesis is largely dependent on methylation and is an important nutrient required for dopamine synthesis and healthy thyroid function. 5-MTHF helps convert phenylalanine to tyrosine which subsequently converts to the neurotransmitters dopamine, adrenaline and noradrenaline. Thyroid balance and energy levels are closely related, and research suggests that normalising thyroid function can positively affect homocysteine levels.12,13 Finally, during the conversion of homocysteine, a molecule of adenosine is produced as a by-product that is involved in the eventual production of ATP.
5-MTHF and methylation: hormone & neurotransmitter synthesis
5-MTHF is a cofactor for an enzyme called biopterin. Biopterin is a cofactor for enzymes used in the synthesis of certain neurotransmitters and hormones that have an influence on mood, motivation, the stress response, and adrenal function. It is crucial for the conversion of tryptophan to 5-HTP, serotonin and melatonin to support mood and sleep as well as having a neuroprotective role, helping to protect nerve cells from heavy metals and toxins.14
Poor methylation can therefore lead to imbalances in neurotransmitter synthesis and breakdown, affecting a person’s mental health, motivation, and energy levels. Stress, high cortisol levels, blood sugar imbalances, and circadian rhythm disruptions can all impact the hypothalamic-pituitary-adrenal axis (HPA axis), nervous system, and adrenal glands, putting additional strain on methylation and contributing to poor energy production.
What can influence methylation? 1-5
Methylation can be highly responsive to external and internal changes, including environmental toxins, poor nutrient intake, stress levels, and genetic SNPs.
Nutrition
Low intakes of nutrient co-factors such as choline, folate, B2, B6, and B12 and the minerals zinc, magnesium, and iron can disrupt methylation. In order for the methylation cycle to work effectively, nutrients must be converted into their active form in the body. For example, B12 as cyanocobalamin must be converted to methylcobalamin, if it is not directly supplemented as such, whilst folic acid (found in fortified foods and most high street supplements) must be converted into the 5MTHF form.15,16 This latter form of folate has been found to be three times more bioavailable than standard folic acid.17 Certain genetic SNPs can greatly affect this conversion, making this an important consideration when advising supplements to clients.
A diet of highly processed foods, high sugar intake, excessive alcohol, and smoking can all reduce nutrient absorption and negatively affect methylation by interfering with nutrient absorption and upregulating their degradation.
Lifestyle
Chronic stress, hormonal imbalance, poor sleep, environmental and chemical toxins including heavy metals and endocrine disruptors, and medications can all interfere with methylation. Other factors such as sub-optimal digestion, proton pump inhibitor (PPI) use, and other medications, can also affect nutrient absorption and reduce levels of essential co-factors.
Gut health, as always, is another important consideration concerning both methylation and energy production. Dysbiosis, low stomach acid production, and low levels of B6 and zinc (needed for stomach acid production), as well as PPI medication, can impair nutrient absorption, especially B12. Reduced protein digestion can also directly affect methionine levels.
Genetics 6, 16, 17
Certain genetic polymorphisms can impact methylation and subsequent energy production. For example, as mentioned, methylation needs folate in its active form 5-methyltetrahydrofolate (5-MTHF) which is usually converted (although poorly) from folic acid. The MTHFR gene variant is a genetic mutation that reduces the efficiency of the normal conversion of folic acid to the bioactive folate, an important methyl donor.
In this instance, there is an increased importance of using a form of folate called methylfolate, which can bypass the MTHFR enzyme. As you can see from the diagram, the bioactive folate donates a methyl group to the amino acid homocysteine, which is then able to convert into methionine, which further goes on to form SAMe.
The methionine and folate cycle are linked via an enzyme called 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR). MTR accepts a methyl group from the folate cycle and donates it to B12 which results in methylated B12, ready for use in its role of the remethylation of homocysteine and the conversion to both methionine and SAMe. SNPs here can slow this process and interfere with the cycle. B12 once used by MTR needs to be reactivated via methylation which is done by the methionine synthase reductase (MTRR) enzyme. This enzyme is specifically impacted by heavy metal toxicity and results in reduced levels of methylated B12, making supporting methylation and reducing heavy metals paramount. Supplementing methylated B12 and zinc as a cofactor can also support MTR.
While MTHFR may be most commonly known by practitioners, there are a whole host of other genetic mutations that can affect the methylation cycle, mood and energy levels, such as MTR as mentioned above. Another example is Dihydrofolate Reductase (DHFR) which supports the synthesis of the neurotransmitters and plays a role in DNA synthesis and repair, however genetic SNPs here increase the risk of folate deficiency.
How can I support methylation?
Nutrition
A nutrient-rich anti-inflammatory diet, rich in an abundance of fruits and vegetables and including good quality protein is essential for optimal methylation. It is advised to limit sugar and refined carbohydrates, which contribute to blood sugar dysregulation, alongside limiting alcohol and caffeine as these can deplete B vitamins and increase stress hormones, which all put additional strain on methylation.
Specific foods to focus on to support methylation include:
- Folate – food sources include spinach and other leafy greens, asparagus, citrus fruits, broccoli, legumes, beetroot, nuts and seeds
- B12 – is mainly found in animal products but many people are deficient in this important nutrient and may need to supplement, especially vegetarians and vegans
- B6 – is found in whole grains, nuts and seeds, legumes, meat and garlic
- Choline – is found in eggs, meats, shitake mushrooms, legumes and cruciferous vegetables
- Betaine – is found in beetroot, spinach, quinoa, whole grains and meat
- B2 – is found in organ meats dairy, eggs, asparagus, broccoli, and spinach
- Choline is an important found in eggs, beans, legumes and cruciferous vegetables
- Minerals zinc and magnesium are important cofactors – include foods rich in zinc such as meat, seafood, legumes and pumpkin seeds and magnesium-rich foods such as green leafy vegetables, nuts, seeds and wholegrains. Or consider magnesium supplementation – especially if there is a high stress load
- Pre and probiotics – including plenty of fruits and vegetables, as well as fermented foods such as kimchi, kefir and sauerkraut can support the microbiome and short-chain fatty acid production. Certain bacteria strains including lactobacillus and bifidobacterium support the production of various nutrients, including folate19
Plant phytochemicals including catechins found in green tea, curcumin, resveratrol, sulforaphane, isothiocyanates in cruciferous vegetables, and allium vegetables, have been proposed in research to affect epigenetic regulatory mechanisms.20
Supplements to support methylation
Folic acid as a dietary supplement is a synthetic compound that requires reduction by the dihydrofolate reductase (DHFR) in the liver, however, this action is relatively weak and when paired with SNPs that affect MTHFR for example, this may lead to a build-up of unmetabolized folic acid and reduced methylation capacity. This leads practitioners to opt for the preferred form of 5-MTHF.16,17 Similarly, methylcobalamin is the active form of B12 and therefore the preferred supplemental form of B12.
As mentioned, methylation is a complex process, and there is not a one-size-fits-all approach. Methylated nutrients can be utilised as one way to support a client’s methylation if appropriate, especially if genetic SNPs are detected.
Lifestyle
- Stress management – incorporate gentle exercise, yoga and meditation. Research has found that yoga and meditation can reduce stress, anxiety, and depression, improve physical and psychological health, and may have influences on gene expression and epigenetic modifications21
- Minimise exposure to environmental toxins from plastics, cigarette smoking, and cleaning and body care products. Switch to natural alternatives and avoid plastics where you can, to reduce the load on the system
- Invest in a good water filter
- Opt for organic produce to minimise pesticide residue
- Improve your sleep schedule and aim for 7-9 hours of good quality sleep at night
- Support detoxification through sauna therapy and liver-supportive foods and herbs
- Regular exercise supports mitochondrial biogenesis to improve energy production
Summary
In summary, methylation is an essential biological process that supports overall cellular function and therefore plays a role in energy production. While this process sounds complicated, it doesn’t need to be in practice! As methylation can be affected by both our external and internal environment, it is important to take a holistic view when supporting clients, taking into consideration diet, supplements, lifestyle, toxic load, sleep and stress.
Key takeaways
- Methylation is a key enzymatic process that occurs billions of times a second in every human cell and is important for the proper function of all body systems.
- Methylation requires methyl donors which are nutrients that play an important role in single-carbon metabolism as they can donate a methyl group to another compound, altering its activity.
- Methylation itself produces various substances needed for optimal energy production, including COQ10 and carnitine.
- Poor methylation can therefore lead to imbalances in neurotransmitter synthesis and breakdown, affecting a person’s mental health, motivation, and energy levels.
- Methylation can be highly responsive to external and internal changes, including environmental toxins, poor nutrient intake, stress levels, and genetic single-nucleotide polymorphisms (SNPs).
- Low intakes of nutrient co-factors such as choline, folate, B2, B6, and B12 and the minerals zinc, magnesium, and iron can disrupt methylation.
- A nutrient-rich anti-inflammatory diet, rich in an abundance of fruits and vegetables, including good quality protein, is essential for optimal methylation.
- Chronic stress, hormonal imbalance, poor sleep, environmental and chemical toxins including heavy metals and endocrine disruptors, and medications can all interfere with methylation.
- Methylcobalamin, is the active form of B12 and therefore the preferred supplemental form of B12.
- Methylation can be affected by both our external and internal environment, it is important to take a holistic view of when supporting clients.
References
- Menezo Y, Clement P, Clement A, Elder K. Methylation: An Ineluctable Biochemical and Physiological Process Essential to the Transmission of Life. Int J Mol Sci. 2020;21(23):9311. Published 2020 Dec 7. doi:10.3390/ijms21239311
- Pizano, J.M., Williamson, C.B. (2020). Nutritional Influences on Methylation. In: Noland, D., Drisko, J., Wagner, L. (eds) Integrative and Functional Medical Nutrition Therapy. Humana, Cham. https://doi.org/10.1007/978-3-030-30730-1_18
- Kennedy DO. B Vitamins and the Brain: Mechanisms, Dose and Efficacy–A Review. Nutrients. 2016 Jan 27;8(2):68. doi: 10.3390/nu8020068. PMID: 26828517; PMCID: PMC4772032.
- 1.Wang Z, Zhu W, Xing Y, Jia J, Tang Y. B vitamins and prevention of cognitive decline and incident dementia: a systematic review and meta-analysis. Nutrition Reviews. 2021;80(4). doi:https://doi.org/10.1093/nutrit/nuab057
- Hara J, Shankle WR, Barrentine LW, Curole MV. Novel Therapy of Hyperhomocysteinemia in Mild Cognitive Impairment, Alzheimer’s Disease, and Other Dementing Disorders. J Nutr Health Aging. 2016;20(8):825-834. doi:10.1007/s12603-016-0688-z
- Jiang W, Xu J, Lu XJ, Sun Y. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2016;21(6):675-685. doi:10.1080/13548506.2015.1120327
- McCaddon A. Vitamin B12 in neurology and ageing; clinical and genetic aspects. Biochimie. 2013;95(5):1066-1076. doi:10.1016/j.biochi.2012.11.017
- Bahtiri E, Islami H, Rexhepi S, Hoxha R. Relationship of homocysteine levels with lumbar spine and femur neck BMD in postmenopausal women. Acta Reumatologica Portuguesa. 2015;40(4):355-362. Accessed December 23, 2024. https://www.researchgate.net/publication/276849846_Relationship_of_homocysteine_levels_with_lumbar_spine_and_femur_neck_BMD_in_postmenopausal_women
- Tardy AL, Pouteau E, Marquez D, Yilmaz C, Scholey A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients. 2020 Jan 16;12(1):228. doi: 10.3390/nu12010228. PMID: 31963141; PMCID: PMC7019700.
- Camfield DA, Wetherell MA, Scholey AB, et al. The effects of multivitamin supplementation on diurnal cortisol secretion and perceived stress. Nutrients. 2013;5(11):4429-4450. Published 2013 Nov 11. doi:10.3390/nu5114429
- Fišar Z, Hroudová J. CoQ10 and Mitochondrial Dysfunction in Alzheimer’s Disease. Antioxidants (Basel). 2024;13(2):191. Published 2024 Feb 2. doi:10.3390/antiox13020191
- 1.Diekman MJM, Van Der Put NM, Blom HJ, Tijssen JGP, Wiersinga WM. Determinants of changes in plasma homocysteine in hyperthyroidism and hypothyroidism. Clinical Endocrinology. 2001;54(2):197-204. doi:https://doi.org/10.1046/j.1365-2265.2001.01170.x
- Yang R, Pu D, Tan R, Wu J. Association of methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms (C677T and A1298C) with thyroid dysfunction: A meta-analysis and trial sequential analysis. Arch Endocrinol Metab. 2022;66(4):551-581. doi:10.20945/2359-3997000000471
- Fanet H, Capuron L, Castanon N, Calon F, Vancassel S. Tetrahydrobioterin (BH4) Pathway: From Metabolism to Neuropsychiatry. Curr Neuropharmacol. 2021;19(5):591-609. doi:10.2174/1570159X18666200729103529
- Spence JD. Nutrition and Risk of Stroke. Nutrients. 2019 Mar 17;11(3):647. doi: 10.3390/nu11030647. PMID: 30884883; PMCID: PMC6470893.
- Menezo Y, Elder K, Clement A, Clement P. Folic Acid, Folinic Acid, 5 Methyl TetraHydroFolate Supplementation for Mutations That Affect Epigenesis through the Folate and One-Carbon Cycles. Biomolecules. 2022;12(2):197. Published 2022 Jan 24. doi:10.3390/biom12020197
- Raghubeer S, Matsha TE. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients. 2021;13(12):4562. Published 2021 Dec 20. doi:10.3390/nu13124562
- Miraglia N, Agostinetto M, Bianchi D, Valoti E. Enhanced oral bioavailability of a novel folate salt: comparison with folic acid and a calcium folate salt in a pharmacokinetic study in rats. Minerva Ginecol. 2016;68(2):99-105.
- Rossi M, Amaretti A, Raimondi S. Folate Production by Probiotic Bacteria. Nutrients. 2011;3(1):118-134. doi:https://doi.org/10.3390/nu3010118
- Thakur VS, Deb G, Babcook MA, Gupta S. Plant phytochemicals as epigenetic modulators: role in cancer chemoprevention. AAPS J. 2014;16(1):151-163. doi:10.1208/s12248-013-9548-5
- Giridharan S. Beyond the Mat: Exploring the Potential Clinical Benefits of Yoga on Epigenetics and Gene Expression: A Narrative Review of the Current Scientific Evidence. Int J Yoga. 2023;16(2):64-71. doi:10.4103/ijoy.ijoy_141_23
All of our blogs are written by our team of expert Nutritional Therapists. If you have questions regarding the topics that have been raised, or any other health matters, please do contact them using the details below:
nutrition@cytoplan.co.uk
01684 310099
Find out what makes Cytoplan different
Last updated on 5th February 2025 by cytoffice



I do have the MTHFR gene variant and am taking Methly Factors. I am also interested in the relationship between this and liver function specifically NAFL – which seems to also be an issue with poor methyalation. Interested on thoughts please.
Poor methylation can impact bile flow and fat metabolism and contribute to higher levels of oxidative stress and inflammation, which all have an impact on liver health. If you would like more individual support from our Nutrition team, please do get in touch at nutrition@cytoplan.co.uk
A very useful well presented set of information. Particularly useful to those with chronic fatigue syndrome/ ME.
Hello Eoin,
Thank you for your kind comment, we are really glad to hear that the article was so useful to you.
This was such an insightful read! I hadn’t realized how much of an impact methylation has on everything from energy levels to overall well-being. You explained the science in a way that’s really easy to follow, even for someone without a background in biology. It’s fascinating how something so cellular can have such wide-reaching effects. Thanks for shedding light on this—definitely motivated to learn more about how to support healthy methylation naturally.